Chapter 1 Introduction
1.1 Eurotransplant
In the 1960s, it was observed that kidney transplantation outcomes were improved when the donor and recipient were siblings with matching leukocyte antigens. Anticipating that leukocyte-antigen compatibility would also improve the outcomes of deceased-donor kidney transplantation, the Dutch immunologist Jon van Rood proposed an international collaboration he named Eurotransplant, in which kidneys from deceased-organ donors would be allocated to kidney transplant candidates on the basis of leukocyte-antigen matching [1]. In 1967, the first kidney was transported internationally, by army helicopter from Leuven, Belgium, to Leiden, the Netherlands, which represents the symbolic start of Eurotransplant [2]. Over the decades that followed, the scope of Eurotransplant broadened beyond the exchange of kidneys, starting with the allocation of livers in the 1970s, followed by pancreases and hearts in the 1980s, lungs in 1988, and intestines in 1999 [3].
In 2025, eight European countries participate in Eurotransplant: Austria, Belgium, Croatia, Germany, Hungary, Luxembourg, the Netherlands and Slovenia. In the 58-year history of Eurotransplant, more than 190,000 donors have been reported to the organization [4]. With the deceased-donor organs that become available, some 6,000 transplantations are performed per year [5]. However, persistent donor shortages also mean that not all patients can be helped in time. In fact, more than a thousand patients die each year while waiting for a transplant [6].
When a donor is reported, Eurotransplant is responsible for the allocation of the available organs. This means that Eurotransplant has to find suitable recipients from the 13,500 candidates who wait for a transplant in the 76 transplantation programs that are active in the region [4]. Ideally, Eurotransplant completes the allocation procedure prior to the explantation of the available organs, which can mean that Eurotransplant has to find suitable recipients within six hours of the reporting of the donor. Completing the allocation within this time window can be challenging, as most donors have multiple organs available for transplantation, donors can be reported at any time of the day, and the transplant centers frequently decline the organs that are offered to their patients.
The central problem in organ allocation is to determine to which candidates the available organs should be offered. In this thesis, we study this allocation question for the allocation of the liver and the kidneys in Eurotransplant. Eurotransplant’s daily operations currently depend on allocation systems that are the product of almost sixty years of scientific, legal, and ethical discussions on allocation. In this first chapter, we describe the background of these systems, and discuss how they have been shaped by objective medical criteria and considerations of fairness.
1.2 Objective medical criteria for organ allocation
In the 1990s, the Netherlands, Belgium and Germany introduced legal frameworks that govern the prioritization of candidates for transplantation. These frameworks required that the allocation process is (i) transparent and (ii) based on medical criteria, such as the medical urgency of the patient and their prospects of transplant success [7], [8]. To meet these requirements, Eurotransplant updated its allocation systems in the 1990s [7]. In scoring these medical criteria, Eurotransplant relies solely on “objective” medical criteria, which are measurable clinical data such as laboratory results or leukocyte antigen matches. Criteria that depend on individual clinical judgment are not used for allocation [8].
These transplantation laws did not specify how these medical criteria ought to be operationalized. In the transplantation literature, a distinction is often made between three principles: medical urgency, medical utility, and transplant benefit [9]. Which principle is used to prioritize candidates affects which candidates have access to transplantation, as we illustrate in Figure 1.1 for three hypothetical candidates: candidate A, B, and C. For medical and historical reasons, the specific principle that is used for allocation differs by organ.
The first principle, medical urgency (or medical need), gives priority to the candidate who would be expected to die first without an organ transplantation. In our hypothetical example, an allocation based on medical urgency would prioritize candidate A because they die first without a transplant. Eurotransplant uses this “sickest-first” principle to allocate livers, with candidates ranked using Model for End-Stage Liver Disease (MELD) scores. These MELD scores quantify a candidate’s expected 90-day mortality risk based on biomarkers measurable from the candidate’s blood. A potential concern about sickest-first allocation is that organs could be allocated to candidates whose life expectancy would remain short even with a transplant. For example, the transplantation of candidate B may seem preferable to transplantation of candidate A, because candidate B would live much longer with the transplant.
The second principle, which is referred to as “medical utility” in the transplantation literature, allocates the organ to the patient who would live longest with the transplant. Under this utilitarian principle, candidate C would be prioritized over candidates A and B (see Figure 1.1). Leukocyte-antigen matching in kidney allocation is motivated based on medical utility, as this practice has positive effects on the survival of the kidney graft and transplant recipient. A general concern about ranking candidates based on medical utility is that it can lead to the transplantation of relatively healthy patients who have little need for a transplant. In our hypothetical example, it could be argued that candidate B should be prioritized over candidate C, since candidate B has a much greater need for transplantation.
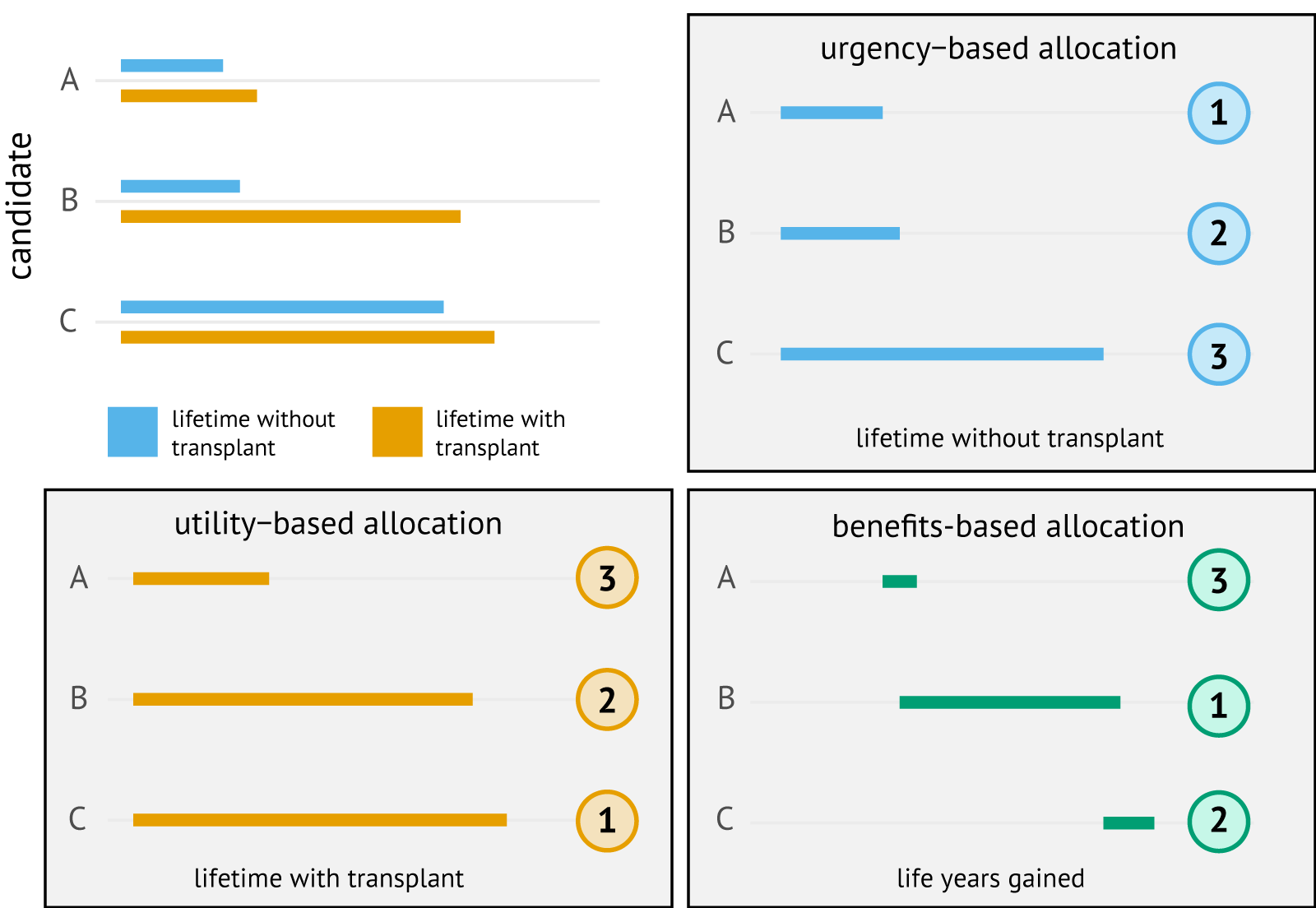
Figure 1.1: Hypothetical scenario in which a choice has to be made between three transplant candidates: candidates A, B, or C. The numbers in circles are the ranks for the hypothetical candidates under urgency-based, utility-based, and benefits-based allocation.
The third principle, transplant benefit, quantifies which candidate is expected to benefit most from receiving the organ. Such benefit is typically operationalized as a contrast between medical utility and medical urgency. For Figure 1.1, we defined transplant benefit as the number of life years gained by transplantation. With this definition of benefit, candidate B would be selected for transplantation by a benefits-based allocation, as they gain the most life years from transplantation. An advantage to such an allocation is that it may prevent the transplantation of candidates who have a short life expectancy after transplantation (candidate A), while also preventing the transplantation of candidates who have little need for a transplant (candidate C). Within Eurotransplant, prioritization of candidates for lung transplantation is based on transplant benefit.
Although an allocation that is based on medical utility, medical urgency, or transplant benefit is conceptually straightforward, applying these principles for organ allocation is complex. One issue is that the lifetime of transplant candidates with or without the transplant is not known to Eurotransplant. Allocation therefore relies on statistical models that quantify a candidate’s expected survival.
1.3 Fairness in organ allocation
Organs are thus allocated in Eurotransplant based on objective medical criteria. However, prioritizing candidates by solely ranking them on these objective medical criteria leads to unfair or unjust waiting list outcomes. For instance, such an allocation would disadvantage candidates with blood group O, who rely on blood group O donors for access to transplantation, while blood group AB patients can accept organs from any blood group. With the aim of making allocation more fair, Eurotransplant has implemented fairness mechanisms in its allocation systems.
One type of fairness mechanisms are the balancing mechanisms that regulate the international exchange of organs among Eurotransplant’s member countries. These balancing mechanisms were introduced after it was observed that the kidney procurement and kidney transplant rates were “totally out of balance” between Eurotransplant’s member countries, which was deemed unfair to countries with high organ donation rates [10]. Their continued importance is underscored by the fact that donor procurement rates still vary widely between the member countries. For example, Belgium reported approximately 30 deceased-organ donors per million people per year over the last decade, while Germany reported only 10 [11]. It is deemed just that Belgian patients benefit from the high Belgian donation rate, and Eurotransplant is even legally required under the Belgian transplantation law to guarantee a “reasonable balance” in the number of organs imported to and exported from Belgium [12]. Such a reasonable balance is ensured by the balancing mechanisms.
Other fairness mechanisms were implemented to ensure access to transplantation for specific patient groups [13]. For example, Eurotransplant’s liver allocation system includes ABO blood group rules which give patients access to roughly the same number of potential donors, regardless of their ABO blood group. This ensures that no patients are disadvantaged by their own blood group [14]. Mechanisms are also in place to facilitate access to transplantation for candidates for whom a suitable donor is difficult to find. For example, in kidney allocation, candidates with difficult-to-match leukocyte-antigen typings receive additional points [15].
A separate issue is that some patient groups may be underserved by how medical urgency, medical utility, or transplant benefit have been defined in Eurotransplant’s allocation systems. For example, several groups of liver transplant candidates are not at risk of an imminent waiting list death, which is what is scored by MELD. These candidates may nonetheless require access to transplantation, for example because of quality of life concerns, or risk of disease irreversibility. To help such candidates access transplantation, the liver allocation system awards exception points to these patient groups. In kidney allocation, prioritization is based on medical utility because patients with end-stage renal disease can survive for prolonged periods of time on dialysis. However, some candidates may lose access to dialysis, and Eurotransplant has implemented rules that facilitate rapid access to transplantation for such candidates.
There are also patients who may deserve access to transplantation based on ethical grounds. In Eurotransplant, children have been given special attention. Another group consists of patients who lose their graft shortly after an initial transplantation procedure. Special rules facilitate access to a repeat transplant for these patients.
Considerations of fairness are thus integral to Eurotransplant’s current allocation systems. The importance of fairness was also underscored by the 2007 Joint Declaration that was signed by the Ministers of Health of Eurotransplant’s member countries, which states that maximizing equality of opportunity for patients is the “most important factor for allocation” [16]. Nevertheless, achieving fairness in organ allocation is not a simple task; in describing Eurotransplant’s kidney allocation system, former medical director Guido Persijn wrote that an allocation system in which transplantation is equally accessible for all patients is “very difficult to implement in practice”, with the implemented allocation systems merely an “attempt” towards this goal [8].
It is thus not surprising that Eurotransplant regularly identifies flaws in its allocation systems, and revises its systems accordingly. A recent example was the “blood group O problem” in kidney allocation [17], which arose because Eurotransplant allowed the transplantation of blood group O kidneys into non-O candidates in case of a perfect leukocyte-antigen match. Because of this rule, blood group O candidates, who cannot accept non-O kidneys because of blood group incompatibility, accumulated on the waiting list. As a result, blood group O candidates faced higher mortality rates than non-O candidates (13% vs 8%) and waited two years longer for a transplant [17]. This problem was addressed in 2010 by also requiring blood group identity for perfectly matched donors. Since then, the gap in waiting times between the blood groups has shrunk, although blood group O candidates continue to experience longer waiting times than blood groups A and AB [18]. The central role of fairness in allocation motivated the first goal of this thesis, which is to study questions related to equality of opportunity in Eurotransplant’s kidney and liver allocation systems. Specifically, in Chapter 4 we study why females are more likely than males to have an adverse waiting list outcome in liver transplantation, and in Chapter 7 we study how immunization affects access to kidney transplantation. These chapters confirm that these patient groups are disadvantaged in the current allocation systems.
1.4 Challenges in improving organ allocation systems
The previous sections discussed that Eurotransplant’s allocation systems have room for improvement. The primary forums to discuss potential improvements to the allocation systems are Eurotransplant’s organ-specific advisory committees, whose members are medical specialists who are affiliated with active transplantation programs in Eurotransplant. These committees can submit recommendations to the Board of Eurotransplant and national competent authorities on how allocation may be improved. Before such recommendations are implemented, approval from these bodies is required.
Changing the allocation systems is a slow process, despite regular meetings of the advisory committees. One challenge is the complexity of the allocation procedure in Eurotransplant. When a donor becomes available, Eurotransplant runs computer algorithms against a central database to generate organ-specific match lists. The transplant centers register their candidates in this database, while donor procurement organizations register donors in this database. The candidates who are eligible for the organ offer appear on the match lists, and the order in which they appear determines the sequence in which Eurotransplant offers the organs to candidates. Improving the allocation system may therefore seem as simple as refining the match list order.
However, discussions on the match list order are not straightforward as match lists behave very differently from regular queuing systems. For example, being highly ranked on the current match list does not guarantee that a candidate will attain a similar rank on the next match list. The top-ranked candidate is also often not transplanted, as the transplant centers regularly decline organ offers because of concerns about the quality of the donor, logistical reasons, or temporary non-transplantability of their patient.
Additional challenges arise from the fact that Eurotransplant’s member countries have different priorities and needs in allocation, in large part because of the international variation in organ donation rates. For example, Belgium has more than twice as many organ donors per million people as Germany. With these varying donation rates, it is often difficult to reach consensus on which patient groups deserve additional attention. Even when the interests of the member countries are aligned, it is often not clear how much additional support a patient group would need to realize equality of opportunity.
Due to these challenges, Eurotransplant’s allocation systems evolve only slowly in response to developments in the field. In fact, the core components of Eurotransplant’s liver and kidney allocation systems have changed little over the past two decades. For example, kidney allocation in Eurotransplant is still based on a point system that was introduced in 1996 despite substantial changes in the waiting list and donor pool. Liver allocation in Eurotransplant has been based on MELD since 2006, while other regions have switched to different allocation mechanisms for the liver (e.g., [19], [20]).
To advance, Eurotransplant requires tools which can give insight into the adequacy and unintended consequences of proposed policy changes. This motivated the second goal of this thesis, which is to develop discrete-event simulation software for this purpose. These simulators replicate the kidney and liver allocation procedures in Eurotransplant, and were developed in close collaboration with relevant stakeholders. In Chapters 5 and 8, we describe and validate simulators for the liver and kidney, respectively, and we demonstrate their usefulness through clinically motivated case studies.
1.5 Outline of this thesis
The thesis is divided into two parts. In Part I, we focus on the allocation of the liver. In Chapter 2, we describe the history of Eurotransplant’s liver allocation system and identify potential areas for improvement. In Chapter 3, we propose a method that can be used to revise MELD more efficiently. In Chapter 4, we describe sex disparity in liver waiting list outcomes in Eurotransplant and study the mechanisms through which this disparity arises. In Chapter 5, we describe and validate the ELAS simulator, a discrete-event simulator tailored to Eurotransplant.
In Part II, we turn to allocation of the kidneys. Chapter 6 outlines the kidney allocation programs used by Eurotransplant, describes their historical development, and discusses contemporary challenges in kidney allocation. In Chapter 7, we examine how having a pre-existing sensitization against HLA antigens affects a candidate’s relative transplant rate. In Chapter 8, we present the ETKidney simulator, a discrete-event simulator for kidney allocation in Eurotransplant.
We reflect on the findings and contributions of this thesis in Chapter 9.